Pacemakers are devices that can be placed in your body, usually by surgery, to support the electrical system in your heart. They can stabilize abnormal heart rhythms and prevent problems that can disrupt or endanger your life.
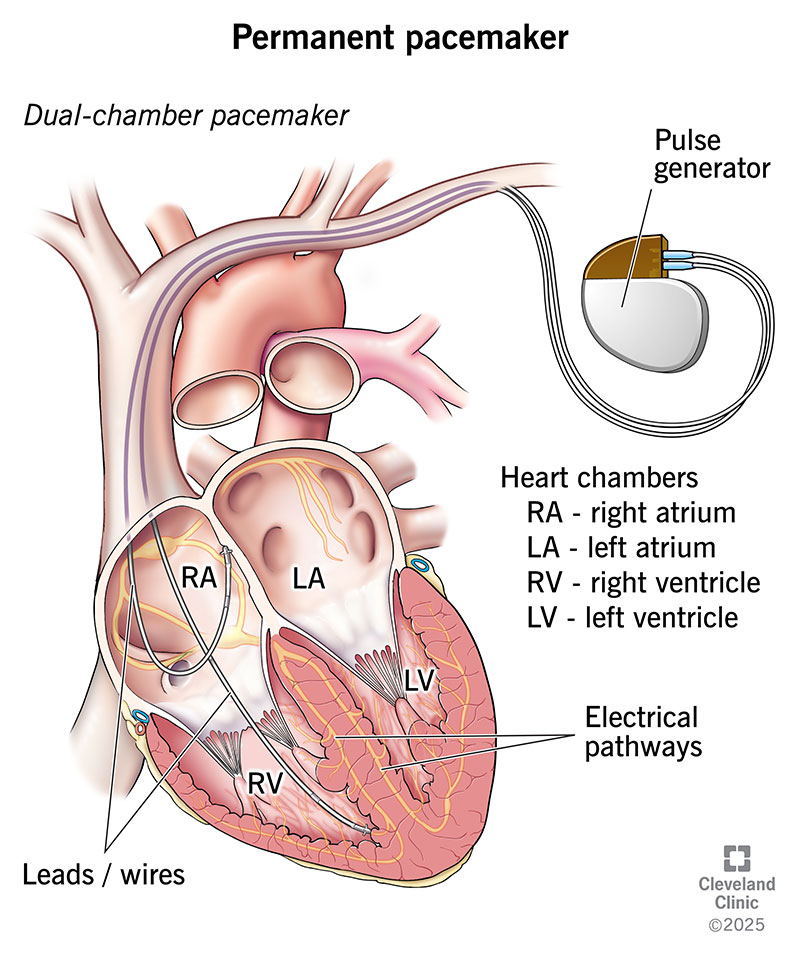
Pacemakers are devices that can be placed in your body, usually by surgery, to support the electrical system in your heart. They can stabilize abnormal heart rhythms and prevent problems that can disrupt or endanger your life.
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Your heart has its own electrical system, which tells your heart’s chambers when it’s their turn to squeeze. When your heart’s electrical system malfunctions, your heart’s chambers may squeeze in the wrong order or squeeze too weakly to provide enough blood to your body. Pacemakers use electrical impulses to correct these kinds of malfunctions.
Conditions that are treatable with a pacemaker include (but aren’t limited to):
Talking to your healthcare provider about your concerns or symptoms is the first step to determining if you have health problems that can be treated with a pacemaker. Some of the symptoms you should tell your healthcare provider about include:
Depending on the heart problem, a specific type of pacemaker — with anywhere from one to three wires (called leads) — may be used. Types of pacemakers include:
Your healthcare provider may recommend a similar device called an implantable cardioverter defibrillator (ICD). Though it isn’t a pacemaker, these are often used with related heart conditions like ventricular tachycardia and ventricular fibrillation.
A pacemaker delivers electrical impulses to control the rhythm of your heart, but it can’t deliver a shock to correct an arrhythmia.
Most new implantable cardioverter defibrillators (ICDs) can do the same job as a pacemaker, as well as detect dangerous heart rhythms. Once these are detected, the ICD can deliver a shock to restore your heart back to its normal rhythm.
Depending on the type of pacemaker used, you’ll undergo a catheter-based, vein-based or surgical-based approach. Advances in surgical knowledge mean these procedures have been refined (improved). The goal is to help you feel less pain, recover faster and get back to your life sooner.
Catheter-based procedures take about an hour or less. The transvenous and surgical-based approaches take between two and five hours. Your healthcare provider will explain which is best for you.
Pacemakers are meant to improve your quality of life and prevent disruptions caused by heart problems. Benefits include:
Pacemaker procedures tend to have few complications, which you can discuss with your healthcare provider. In general, the following complications are possible:
While it depends on the specific model of pacemaker and how often it has to assist your heart, pacemakers are now available that can last as long as 10 or 15 years. Your healthcare provider can tell you the average lifespan of the device you’ll receive, and will also schedule follow-up appointments to check your pacemaker’s battery level. It’s also usually a simpler process to replace a pacemaker battery than it was to implant the device in the first place.
The life expectancy of a person who has a pacemaker depends on several factors, especially a person’s age when they have a pacemaker implanted and their health conditions. People who have fewer or less-severe health concerns tend to live longer and are more likely to have a normal or near-normal life expectancy.
If you have a pacemaker implanted, it’s best to manage your health and be aware of how you feel.
A note from Cleveland Clinic
Heart problems that result in needing a pacemaker can cause you to feel stressed, anxious or scared. Your healthcare provider can help you understand your situation and talk you through it so you can feel better about what’s happening. Your healthcare provider can also recommend resources that can help you cope with any questions or concerns you might have.
Last reviewed by a Cleveland Clinic medical professional on 02/28/2022.
Learn more about our editorial process.
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy