Advertisement
FDA-approved nerve stimulator helps open your airway
If you have obstructive sleep apnea, your airway tends to collapse when you sleep. After snoring, gasping and choking your way through the night, you’re getting by on little rest the next day.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The most common solution is CPAP (continuous positive airway pressure). While your partner may appreciate quieter nights, chances are you’re not a big fan.
“It’s the standard treatment for obstructive sleep apnea, but about half the people don’t like it very much,” explains surgical sleep medicine specialist Alan Kominsky, MD.
A Cleveland Clinic study found that just 44% of the patients prescribed CPAP for obstructive sleep apnea were still using it three years later.
That means a significant number of patients are not being treated for obstructive sleep apnea, which can lead to hypertension, stroke and cardiac problems.
Dental appliances can help patients with mild to moderate obstructive sleep apnea. Surgery to remove obstructive tissue is also an option. But it’s invasive and requires an extensive recovery.
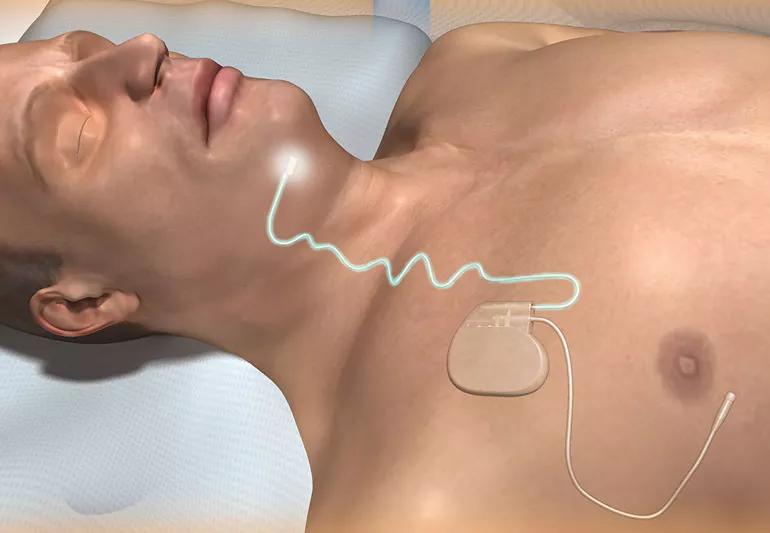
Another option is an implantable, programmable device approved by the U.S. Food and Drug Administration in 2014. The Inspire® upper airway stimulation device looks like a heart pacemaker.
It has two leads. One senses your breathing. The other stimulates the nerve that controls the tongue, to move it forward and open your throat.
“A pivotal trial demonstrated the device’s safety and effectiveness in a small group of patients with moderate to severe obstructive sleep apnea,” says Dr. Kominsky. Five years later, those improvements have been measured and are persistent.
The airway stimulation device is available at select U.S. medical centers, including Cleveland Clinic. To be eligible for it:
Eligible patients must have a recent sleep study. The next step is to perform sleep endoscopy. This outpatient procedure begins with sedation so that doctors can insert an endoscope into the airway. This lets them view the pattern of airway collapse to confirm the device will help.
The stimulator system is then implanted under general anesthesia in an outpatient procedure.
“One month after the procedure, we turn the device on,” says Dr. Kominsky. “One month after that, we perform a repeat sleep study. This helps us make adjustments to ensure the device will deliver the maximum benefit.”
Advertisement
Learn more about our editorial process.
Advertisement

Stress, weight gain and forgetfulness are just a few effects of losing sleep

Ignoring the warning signs could put you at risk for serious health issues

Mouth taping isn’t a recommended treatment for sleep apnea or snoring

Here’s what to know from a sleep medicine specialist

This connection is yet another reason to seek help for OSA

The short answer from a cardiovascular researcher
Sleeping disorder may increase danger of cardiac event

Heroic snoring can be associated with obstructive sleep apnea

Focus on your body’s metabolic set point by eating healthy foods, making exercise a part of your routine and reducing stress

PFAS chemicals may make life easier — but they aren’t always so easy on the human body

While there’s little risk in trying this hair care treatment, there isn’t much science to back up the claims