Advertisement
Adult vaccination has begun, while clinical trials for a children's vaccine are underway
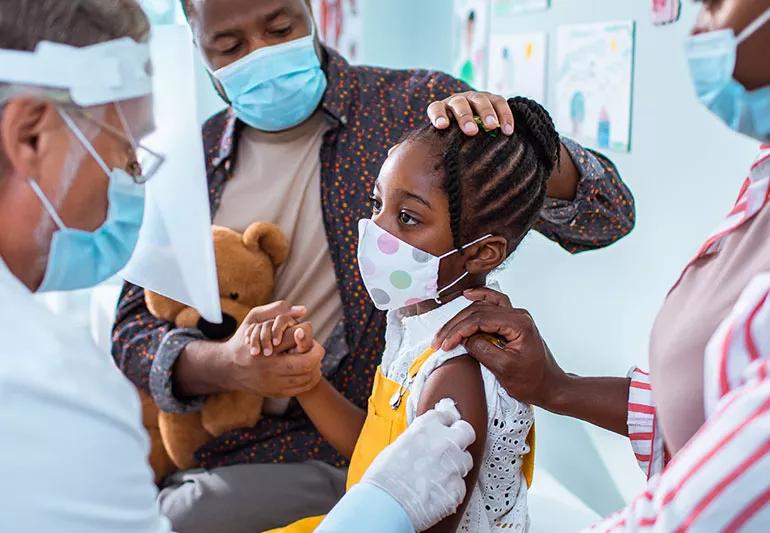
The United States finally has a coronavirus vaccine for adults (three to be exact!). And although this is fantastic news, experts say that children will also need to be vaccinated to get the pandemic under control. Still, clinical trials for a kid’s vaccine are underway, and although it may take another year to receive the data and produce a vaccine, it’s promising news.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Pediatric infectious disease specialist Frank Esper, MD, explains the ins and outs of developing a vaccine for children and why the first COVID-19 vaccines weren’t approved for kids.
“Both adults and children will need to be vaccinated for things to return to some sort of normalcy,” explains Dr. Esper. “Many vaccine manufacturers have now started research and clinical trials for a vaccine that is safe and effective for kids.”
Kids weren’t involved in the original adult clinical trials because the data tells us that severe illness from COVID-19 tends to happen to adults (especially in older adults). We’re not seeing the same sort of response or sickness in children.
That’s not to say that children are immune to COVID-19, because they aren’t. But they tend to be more resilient and fare better if they get sick, notes Dr. Esper (although a very small number of kids have developed a serious inflammatory condition linked to coronavirus).
One of the reasons for focusing on an adult version first is because when children are involved in clinical trials, there is oftentimes more layers of protection to go through. For instance, the child and both parents typically have to agree to participate in the trial or study.
“Kids are a special and vulnerable population and we try to protect them because they can’t make decisions for themselves,” says Dr. Esper. “In clinical trials – whether for a vaccine or any type of treatment – we don’t want to speed things up because it’s a matter of protection and safety.”
So the first priority was for an adult vaccine, not only because of the outcomes we’re seeing, but because an adult vaccine was faster to create due to the potential risk and rigorous safety precautions needed for the development of a children’s vaccine.
“It’s encouraging to see drug companies expand their vaccine clinical trials to include babies and young children,” says Dr. Esper. “This is a crucial step in our efforts to slow the spread of COVID-19 and move closer towards normalcy.”
Here’s where current pediatric vaccine trials stand:
Advertisement
Since a child’s immune system is different from an adult’s, COVID-19 vaccines for younger children, especially, may require different dosage levels or formulations than the adult versions.
Immune systems in kids can vary greatly depending on age. A 16-year-old is going to have a much different immune system than a 16-month-old. Because of this, additional data and research is needed when evaluating a vaccine for kids.
It’s even true for the flu shot. Babies six months and older should get a flu shot every year, but some kids six months through eight years of age may need two doses for more protection. This is because of differing immune system responses at different ages.
“Children’s immune systems are growing up just as they are,” explains Dr. Esper. “We often split kids up by age groups and stages. We can’t just say all kid’s immune systems are the same at any given age.”
For adults, we’re often lumped together from age 18 to 65 and then 65 and over. With children, there is a much wider range in terms of stages and ages because they are still growing and developing. All of this has to be taken into consideration with a vaccine since kids at varying ages will likely respond differently.
Because of all the challenges with kid’s immune systems and the protection and safety protocols in clinical trials, a COVID-19 vaccine for children will still take some time to develop. However, these pediatric vaccine trials will provide critical safety data and help us better understand the vaccine’s immune response in children.
Until we have a kid’s vaccine, we must remember to follow the safety precautions we’re all now familiar with. This means wearing face masks, washing our hands, avoiding crowds and maintaining physical space from others.
Advertisement
Learn more about our editorial process.
Advertisement

Setting specific expectations and praising good behavior are crucial to cultivating discipline

‘Active shooter’ exercises may raise both awareness and anxiety

Stay calm, don’t give in and try to refocus their attention

Integrating coping skills into your teen’s daily routine helps turn self-care into a lifelong healthy habit

Tantrums and meltdowns are normal, but you can help your child manage their bigger emotions

Most routine vaccines are safe for people living with multiple sclerosis — but be sure to talk with your care team about your needs

The medication is ineffective and — in the case of animal ivermectin — potentially dangerous

Talking in the car, resisting the urge to judge and asking specific questions can help rebuild rapport

Focus on your body’s metabolic set point by eating healthy foods, making exercise a part of your routine and reducing stress

PFAS chemicals may make life easier — but they aren’t always so easy on the human body

While there’s little risk in trying this hair care treatment, there isn’t much science to back up the claims